Should allopurinol be given simultaneously with rasburicase for tumor lysis syndrome?
1
Introduction
Tumor lysis syndrome (TLS) is an oncologic emergency manifesting as acute metabolic derangements with or without downstream organ dysfunction secondary to the release of intracellular contents of lysed tumor cells.1 Tumor cell lysis may occur spontaneously in patients with a high burden of disease characterized by rapid proliferation (such as acute lymphoblastic leukemia), but is more commonly related to administration of systemic or, in some cases, local anticancer therapy. TLS is subclassified as laboratory TLS, defined by laboratory changes alone, and clinical TLS, in which laboratory changes are accompanied by evidence of end-organ damage, in the Cairo-Bishop TLS classification system (Table 1).2 The risk of TLS varies from patient to patient, but is broadly attributable to malignancy characteristics (such as tumors with high rates of proliferation and/or high chemosensitivity), treatment intensity, disease burden, and comorbidities (such as pre-existing renal dysfunction or dehydration, gout with hyperuricemia, or cardiovascular instability).3,4
1
| Table 1. Cairo-Bishop TLS classification.2 | |
|---|---|
| Laboratory TLS: Two or more of the laboratory abnormalities below within 3 days prior to or 7 days after initiation of treatment. | Clinical TLS: Criteria for laboratory TLS and any of the signs of end-organ damage below when they are not attributable to an alternate etiology. |
| Phosphorus ≥ 4.5 mg/dL or ≥ 25% increase from baseline Potassium ≥ 6 mEq/L or ≥ 25% increase from baseline Uric acid ≥ 8 mg/dL or ≥ 25% increase from baseline Calcium ≤ 7 mg/dL or ≥ 25% decrease from baseline | Cardiac arrhythmia/sudden death Creatinine ≥ 1.5x ULN Seizure |
| Abbreviations: TLS=tumor lysis syndrome; ULN=upper limit of normal. |
|
1
Clinical manifestations of TLS are principally related to direct or indirect effects of systemic release of intracellular nucleic acids, phosphorus, and potassium.2 Uric acid (produced via catabolism of intracellular DNA and subsequent metabolism of purine nucleic acids) and/or calcium phosphate (produced as a result of excessive phosphorus release and subsequent binding with systemic calcium) may deposit in the renal tubules, leading to acute kidney injury, which may in turn worsen electrolyte abnormalities. Other manifestations of hyperphosphatemia may include nausea or vomiting, diarrhea, lethargy, or seizures. Hypocalcemia (resulting from the formation of calcium phosphate) may produce muscle spasms, paresthesias, or tetany, confusion or delirium, seizures, or severe arrythmias. Hyperkalemia may also produce gastrointestinal symptoms such as nausea, vomiting, or diarrhea, as well as the potential for severe arrythmias.
The principles of TLS management are centered around assessing and reducing risk and early recognition and reversal of laboratory abnormalities and clinical manifestations.3,4 Key considerations for risk-stratified TLS prevention and initial treatment include hydration, use of xanthine oxidase inhibitors, and/or use of the recombinant urate oxidase rasburicase, along with individualized management strategies for electrolyte derangements. Rasburicase is recommended in consensus guidelines as preventive therapy in patients who are at high risk of TLS, and as treatment of patients with laboratory or clinical TLS characterized by hyperuricemia. The xanthine oxidase inhibitor allopurinol is recommended as prophylaxis in patients at low-to-intermediate risk of TLS, and newer data support the preventive use of the xanthine oxidase inhibitor febuxostat.5
Simultaneous administration of xanthine oxidase inhibitors with rasburicase in the prevention or treatment of TLS is a relatively common clinical practice.6-8 In guidelines for TLS prevention, use of a xanthine oxidase inhibitor alongside rasburicase in patients at high risk of TLS is not mentioned.3 Guidelines for the treatment of TLS do not directly address concomitant use of xanthine oxidase inhibitors and rasburicase, but note that “after initial management with rasburicase, the subsequent use of allopurinol is not necessary.”4
Concerns related to use of xanthine oxidase inhibitors in TLS-related hyperuricemia
Most mammals express urate oxidase, which facilitates the processing of uric acid to form allantoin; the gene encoding uric oxidase is inactive in humans and great apes due to a nonsense mutation developed during hominoid evolution, rendering uric acid the final product of the nucleoside metabolism pathway (Figure 1).9 Rasburicase, a recombinant form of urate oxidase, is the sole pharmacologic method of directly reducing uric acid levels that has been studied for TLS. It is well-recognized that while xanthine oxidase inhibitors prevent the formation of new uric acid from xanthine during nucleoside metabolism, they do not affect uric acid that has already formed; thus, the hypouricemic effect of xanthine oxidase inhibitors relies on renal elimination of uric acid, and onset may be days after initiation.10,11
2
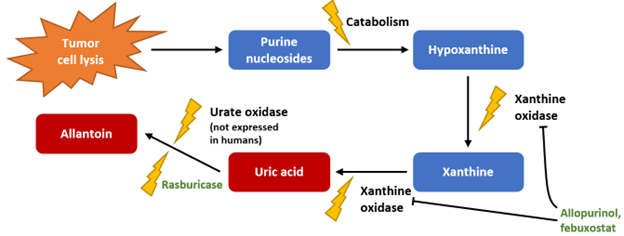
Figure 1. Nucleoside metabolism and the effects of uric acid-lowering agents.
3
The urine solubility of uric acid at physiologic pH is relatively low (Figure 2).1 Preventive use of xanthine oxidase inhibitors in patients at risk of TLS is intended to avoid acute increases in uric acid that exceed urine solubility, thereby reducing the risk of renal injury due to uric acid crystallization and deposition in renal tubules. However, inhibition of xanthine oxidase during TLS results in accumulation of hypoxanthine and xanthine.12,13 While hypoxanthine has relatively high urine solubility, xanthine has the lowest urine solubility of all purine metabolites and, like uric acid, may precipitate to produce renal injury.14 Allantoin, conversely, has very high urine solubility. Thus, in a patient at high risk of TLS or who is experiencing TLS-related hyperuricemia, the use of rasburicase to facilitate rapid breakdown of uric acid and elimination as allantoin may be preferable to the accumulation of xanthine that may occur when a xanthine oxidase inhibitor is used alone or in combination with rasburicase.
4
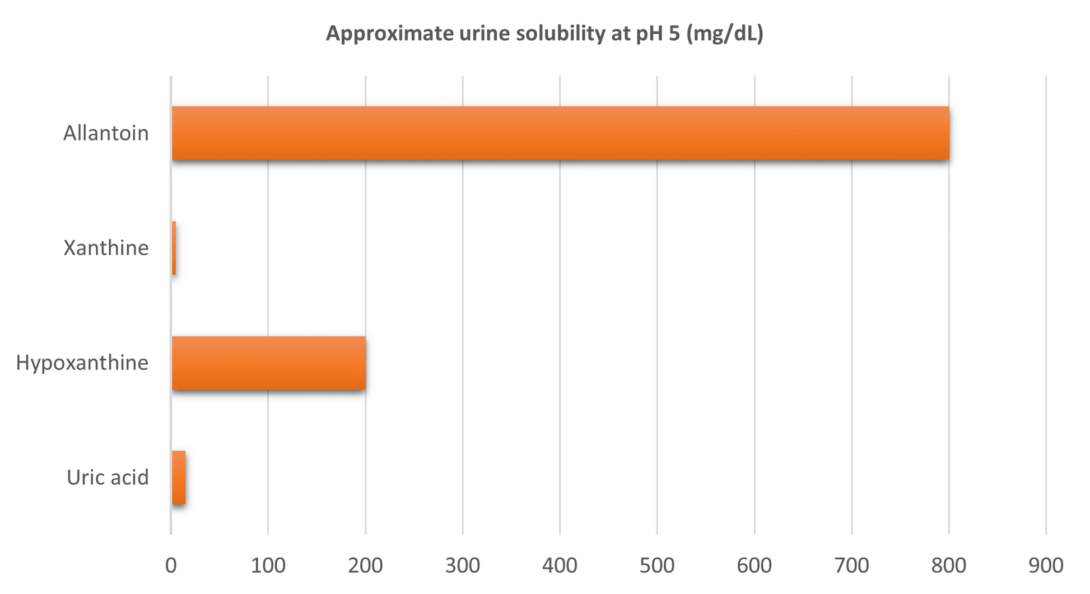
Figure 2. Solubility of purine metabolites in urine at physiologic pH.
5
Xanthine deposition in patients with cancer
Xanthine nephrolithiasis is exceedingly rare in the general population, and is believed to be encountered almost exclusively in patients with hereditary disorders of purine metabolism or who receive xanthine oxidase inhibitors in the setting of hyperuricemia.15,16 Several case studies have described xanthine deposition-related complications, ranging from visible xanthine crystalluria to renal failure resulting in death, in patients receiving a xanthine oxidase inhibitor for prevention or treatment of TLS.17-21 The earliest reported case was in a 23-year-old patient treated for lymphoma in 1969, in whom allopurinol was administered for 4 days to normalize uric acid prior to initiation of chemotherapy; the patient was critically ill at the time of treatment initiation, and died 3 days later.17 Despite low serum and urinary uric acid levels following chemotherapy, kidney injury was noted at the time of death; during autopsy, xanthine calculi were noted in bilateral kidney parenchyma.
In the pre-rasburicase era, 2 prospective studies evaluated changes in urinary excretion of purine metabolites in patients receiving allopurinol as TLS prophylaxis. The first study enrolled 11 patients with newly-diagnosed non-Hodgkin lymphoma requiring systemic treatment; patients received 2 to 5 days of allopurinol prior to initiation of chemotherapy, and continued allopurinol for the duration of the study.22 Daily 24-hour urine collections for analysis of uric acid, xanthine, and hypoxanthine were performed at least 24 hours after initiation of allopurinol (prior to initiation of chemotherapy) and for 4 to 7 days after treatment initiation. Compared to pre-chemotherapy baseline, mean daily excretion of uric acid increased approximately 2-fold for uric acid and approximately 7-fold for both hypoxanthine and xanthine; the largest increases from baseline in daily hypoxanthine and xanthine excretion were 12-fold and 23-fold, respectively. All patients developed crystalluria; samples from 8 patients were morphologically determined to be composed of xanthine, and 3 samples were confirmed to be largely composed of xanthine by chromatography. Four patients developed renal injury after initiation of chemotherapy; 3 of these patients had the highest post-chemotherapy urinary xanthine concentrations among the cohort (ranging from 6- to 27-fold higher concentrations than baseline), and experienced peak serum uric acid levels less than 7 mg/dL.
The second pre-rasburicase study evaluated systemic and urine levels of purine metabolites in 19 children with acute lymphoblastic leukemia who developed TLS while receiving prophylactic allopurinol alongside chemotherapy.23 Compared to pre-chemotherapy baseline, mean daily urinary excretion of uric acid, hypoxanthine, and xanthine were found to increase by 174%, 1630%, and 1460%, respectively, during TLS. Four patients developed renal injury after initiation of chemotherapy; compared to those who did not develop renal injury, these patients were significantly older (mean 10.9 vs 5.25 years) and had significantly higher plasma xanthine levels (mean 9.1 vs 2.33 mg/dL). Conversely, precipitation of xanthine in urine samples occurred at similar rates in patients who did and did not develop renal injury.
No prospective studies designed to evaluate the efficacy or safety of concomitant use of rasburicase and a xanthine oxidase inhibitor relative to use of either agent alone have been performed. A large single-arm study of rasburicase allowed use of allopurinol after treatment with rasburicase was completed, but no patients ultimately received it.24 A phase 3 randomized trial comparing allopurinol and febuxostat in patients at intermediate or high risk of TLS excluded patients intended to receive rasburicase.25 One randomized trial of single fixed doses of rasburicase utilized concomitant allopurinol for 6 days, but only enrolled 24 patients, nearly all of whom had acute myeloid leukemia and may not have been at high TLS risk.3,26
Large randomized trials of rasburicase for patients at high risk of TLS either did not allow concomitant xanthine oxidase inhibitor use or staggered its use to follow treatment with rasburicase.27,28 The latter study compared allopurinol monotherapy for 5 days, rasburicase monotherapy for 5 days, and rasburicase from days 1 to 3 followed by allopurinol from days 3 to 5 in 275 patients at risk of TLS (90% of whom were considered high-risk); patients receiving rasburicase monotherapy were most likely to experience sustained reductions in uric acid to 7.5 mg/dL or lower from days 3 to 7 after initiation of chemotherapy, and median time to uric acid control was 4 hours in the rasburicase monotherapy and combination groups (versus 27 hours in the allopurinol monotherapy group).28
A 2007 case report describes an 11-year-old who developed TLS with oliguric renal failure following initiation of chemotherapy for acute lymphoblastic leukemia despite prophylaxis with allopurinol and rasburicase.20 The patient underwent repeated hemodialysis sessions beginning on day 5 post-treatment initiation and renal ultrasounds demonstrated bilateral renal calculi; 17 days following initiation of therapy she developed acute respiratory failure followed by death attributed to septic shock, cerebral edema, and uncal herniation. During autopsy, multiple stones were found in bilateral renal papillae and pelves, and were found to be composed entirely of xanthine on spectrophotometric analysis.
A 2022 report describes 3 patients with hematologic malignancies who developed TLS while receiving febuxostat prophylaxis.19 Each of these patients developed hyperuricemia alongside other TLS manifestations, and were noted to have crystalluria for 2 to 3 days; analysis of the urine sediment indicated the crystals were composed of xanthine.
Discussion
Although xanthine nephrolithiasis and other xanthine deposition-related complications are rare, they are likely to have increased incidence in patients who experience significant elevations in systemic xanthine concentrations above the limit of its urine solubility. Observational studies indicate such accumulations occur rapidly and with frequency in patients who develop TLS while receiving xanthine oxidase inhibitors.22,23 The scarcity of literature describing xanthine-related renal injury in patients receiving xanthine oxidase inhibitors as prophylaxis for high risk of TLS or while experiencing TLS-related hyperuricemia suggests that the incidence of such events in this population remains relatively low; however, because systemic and urine xanthine levels are not routinely monitored and renal injury and/or crystalluria may be assumed to be related to uric acid, it is also likely that some cases are unrecognized. Data from randomized trials indicate clear superiority of rasburicase to allopurinol in lowering uric acid levels, with no evidence of additional benefit with the use of both agents in patients at high risk of TLS.27,28 Taken together, this information suggests that in a patient in whom rasburicase is indicated as prevention or treatment of TLS, concurrent initiation or continuation of a xanthine oxidase inhibitor does not produce additional benefit and may present undue risk of xanthine-mediated renal injury.
Summary
Small prospective studies indicate that use of xanthine oxidase inhibitors in patients with TLS-related hyperuricemia produces significant increases in systemic and urine xanthine concentrations; these studies and several case reports indicate that such conditions may lead to xanthine-induced renal injury. Given that there is no additional benefit in lowering uric acid with concomitant use of rasburicase and a xanthine oxidase inhibitor versus rasburicase alone, a xanthine oxidase inhibitor should not be administered simultaneously in a patient in whom rasburicase is indicated.
References
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854. doi:10.1056/NEJMra0904569
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11. doi:10.1111/j.1365-2141.2004.05094.x
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586. doi:10.1111/j.1365-2141.2010.08143.x
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767-2778. doi:10.1200/JCO.2007.15.0177
- Spina M, Nagy Z, Ribera JM, et al. FLORENCE: a randomized, double-blind, phase III pivotal study of febuxostat versus allopurinol for the prevention of tumor lysis syndrome (TLS) in patients with hematologic malignancies at intermediate to high TLS risk. Ann Oncol. 2015;26:2155-2161. doi:10.1093/annonc/mdv317
- Hossain S, Naber M, Yacobucci MJ. A retrospective observational study of a low fixed-dose rasburicase protocol for the treatment of tumor lysis syndrome in adults. J Oncol Pharm Pract. 2022;28(6):1326-1331. doi:10.1177/10781552211021147
- Martens KL, Khalighi PR, Li S, et al. Comparative effectiveness of rasburicase versus allopurinol for cancer patients with renal dysfunction and hyperuricemia. Leuk Res. 2020;89:106298. doi:10.1016/j.leukres.2020.106298
- Shaikh SA, Marini BL, Hough SM, Perissinotti AJ. Rational use of rasburicase for the treatment and management of tumor lysis syndrome. J Oncol Pharm Pract. 2018;24(3):176-184. doi:10.1177/1078155216687152
- Yeldandi AV, Yeldandi V, Kumar S, et al. Molecular evolution of the urate oxidase-encoding gene in hominoid primates: nonsense mutations. Gene. 1991;109:281-284. doi:10.1016/0378-1119(91)90622-i
- Krakoff IH, Meyer RL. Prevention of Hyperuricemia in Leukemia and Lymphoma: Use of Alopurinol, a Xanthine Oxidase Inhibitor. JAMA. 1965;193:1-6. doi:10.1001/jama.1965.03090010007001
- Smalley RV, Guaspari A, Haase-Statz S, Anderson SA, Cederberg D, Hohneker JA. Allopurinol: intravenous use for prevention and treatment of hyperuricemia. J Clin Oncol. 2000;18(8):1758-1763. doi:10.1200/JCO.2000.18.8.1758
- Simmonds HA, Cameron JS, Morris GS, Davies PM. Allopurinol in renal failure and the tumour lysis syndrome. Clin Chim Acta. 1986;160:189-195. doi:10.1016/0009-8981(86)90141-5
- Takai M, Yamauchi T, Ookura M, et al. Febuxostat for management of tumor lysis syndrome including its effects on levels of purine metabolites in patients with hematological malignancies – a single institution’s, pharmacokinetic and pilot prospective study. Anticancer Res. 2014;34:7287-7296. https://www.ncbi.nlm.nih.gov/pubmed/25503162
- Seegmiller JE. Xanthine stone formation. Am J Med. 1968;45:780-783. doi:10.1016/0002-9343(68)90210-6
- Mandel NS, Mandel GS. J Urol. In. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. Vol 142. 1989:1516-1521.
- Pais VM, Jr., Lowe G, Lallas CD, Preminger GM, Assimos DG. Urology. In. Xanthine urolithiasis. Vol 67. 2006:1084 e1089-1011.
- Band PR, Silverberg DS, Henderson JF, et al. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. N Engl J Med. 1970;283(7):354-357. doi:10.1056/NEJM197008132830708
- Gomez GA, Stutzman L, Chu TM. Xanthine nephropathy during chemotherapy in deficiency of hypoxanthine-guanine phosphoribosyltransferase. Arch Intern Med. 1978;138(6):1017-1019. doi:10.1001/archinte.138.6.1017
- Ito S, Fujiwara SI, Yoshizawa T, et al. Urine Xanthine Crystals in Hematologic Malignancies with Tumor Lysis Syndrome. Intern Med. 2022;61(21):3271-3275. doi:10.2169/internalmedicine.9332-22
- LaRosa C, McMullen L, Bakdash S, et al. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatr Nephrol. 2007;22(1):132-135. doi:10.1007/s00467-006-0287-z
- Potter JL, Silvidi AA. Xanthine lithiasis, nephrocalcinosis, and renal failure in a leukemia patient treated with allopurinol. Clin Chem. 1987;33(12):2314-2316. https://www.ncbi.nlm.nih.gov/pubmed/3480085
- Hande KR, Hixson CV, Chabner BA. Postchemotherapy purine excretion in lymphoma patients receiving allopurinol. Cancer Res. 1981;41(6):2273-2279. https://www.ncbi.nlm.nih.gov/pubmed/7237428
- Andreoli SP, Clark JH, McGuire WA, Bergstein JM. Purine excretion during tumor lysis in children with acute lymphocytic leukemia receiving allopurinol: relationship to acute renal failure. J Pediatr. 1986;109:292-298. doi:10.1016/s0022-3476(86)80387-0
- Coiffier B, Mounier N, Bologna S, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Groupe d’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. J Clin Oncol. 2003;21:4402-4406. doi:10.1200/JCO.2003.04.115
- Tamura K, Kawai Y, Kiguchi T, et al. Efficacy and safety of febuxostat for prevention of tumor lysis syndrome in patients with malignant tumors receiving chemotherapy: a phase III, randomized, multi-center trial comparing febuxostat and allopurinol. Int J Clin Oncol. 2016;21:996-1003. doi:10.1007/s10147-016-0971-3
- Vachhani P, Baron J, Freyer CW, et al. A phase 2 trial of single low doses of rasburicase for treatment of hyperuricemia in adult patients with acute leukemia. Leuk Res. 2021;107:106588. doi:10.1016/j.leukres.2021.106588
- Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998-3003. doi:10.1182/blood.v97.10.2998
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone–results of a multicenter phase III study. J Clin Oncol. 2010;28:4207-4213. doi:10.1200/JCO.2009.26.8896
Prepared by:
Michael Buege, PharmD, BCOP
Clinical Assistant Professor, Drug Information Specialist
University of Illinois at Chicago College of Pharmacy
March 2023
The information presented is current as February 22, 2023. This information is intended as an educational piece and should not be used as the sole source for clinical decision-making.