What is the optimal sequencing for first-line treatment of BRAF-mutated metastatic melanoma?
Background
Melanoma represents the fifth most common cancer in the United States, accounting for about 5% to 6% of all new cancer diagnoses.1 It affects melanocytes that are predominately located in the basal layer of the epidermis (cutaneous melanoma), but can occur in other tissues (mucosal and uveal melanoma).2,3 While only a small portion (4%) of patients present with metastatic disease, the overall prognosis is quite poor, particularly as compared to localized disease, which has a 99.4% 5-year relative survival rate.4 Fortunately, the treatment landscape for metastatic melanoma has completely transformed in the last decade. Since 2011, 12 single-agent drugs or drug combinations have been approved for patients with metastatic melanoma; subsequently, the 5-year overall survival rate has moved from <10% prior to 2011 to 39% to 52% in recent immune checkpoint inhibitor (ICI) clinical trials.5,6
BRAF V600 mutant disease
For patients presenting with metastatic disease, evaluation of somatic mutations is recommended to inform treatment selection.7,8 Currently, only assessment of BRAF V600 mutations is necessary, though testing for KIT or BRAF non-V600 mutations or a next-generation DNA sequencing panel may be considered.7 BRAF V600 mutations are present in 40% to 60% of cutaneous melanoma cases, with V600E mutations accounting for 80% of BRAF-mutated melanomas (the remaining are V600K mutations).9 The assessment for BRAF V600 mutations is important in directing therapy due to the availability of combined b-Raf proto-oncogene (BRAF) plus mitogen-activated extracellular signal-regulated kinase (MEK) inhibitor therapies, which target the mitogen-activated protein kinase pathway that regulates cell growth, survival, and invasion.8
Guideline-recommended treatment
For metastatic, BRAF-mutated cutaneous melanoma, preferred first-line treatment options include ICIs (nivolumab, nivolumab/ipilimumab, and pembrolizumab) and targeted BRAF/MEK inhibitors (dabrafenib/trametinib, vemurafenib/cobimetinib, and encorafenib/binimetinib); for non-BRAF-mutated disease, only the former is recommended.7,10 Pembrolizumab with low-dose ipilimumab or the triplet combination of vemurafenib, cobimetinib, and atezolizumab may also be considered, but have more limited evidence to support their use over preferred treatment options.7 Current National Comprehensive Cancer Network melanoma guidance does not provide a preference for use of ICIs versus BRAF/MEK inhibitors as front-line systemic therapy. It is noted, however, that ICIs may be associated with more durable disease control, while BRAF/MEK inhibitors may be more appropriate in patients who would benefit from a more rapid response. Further, the guidance notes that nivolumab/ipilimumab combination therapy is associated with increased clinical response rates, progression-free survival (PFS), and overall survival as compared to PD-1 inhibitor monotherapy, but this benefit comes at the cost of increased immune-related adverse effects. Lastly, for patients who experience disease progression, preferred second-line therapies include the same ICIs and BRAF/MEK inhibitors recommended for first-line treatment. Ultimately, treatment decisions are nuanced, individualized, and considerate of a patient’s overall health, the location and extent of metastases, the aggressiveness of the disease, and treatment preferences.
Optimal sequencing
Since both ICIs and BRAF/MEK inhibitors are treatment options for first-line and second-line therapy, the optimal treatment sequence for patients with systemic therapy-naive BRAF V600-mutant metastatic disease is an area of interest. Recent data have been presented that interrogate this optimal sequence: the DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing) trial and the SECOMBIT (Sequential Combo Immuno and Target Therapy) trial.11,12
DREAMseq
The DREAMseq phase 3 trial addressed the optimal sequence of ICIs and BRAF/MEK inhibitors in BRAF-mutated metastatic melanoma.11 Patients with treatment-naïve BRAF V600-mutant metastatic melanoma were enrolled and randomized to receive either nivolumab/ipilimumab or dabrafenib/trametinib until disease progression and were then assigned to the alternative therapy (Figure 1). The nivolumab/ipilimumab treatment arms received nivolumab/ipilimumab induction dosing every 3 weeks for 4 doses followed by nivolumab 240 mg every 2 weeks for up to 72 weeks. The dabrafenib/trametinib arms received dabrafenib 150 mg twice daily plus trametinib 2 mg daily until disease progression. The primary outcome was 2-year overall survival.
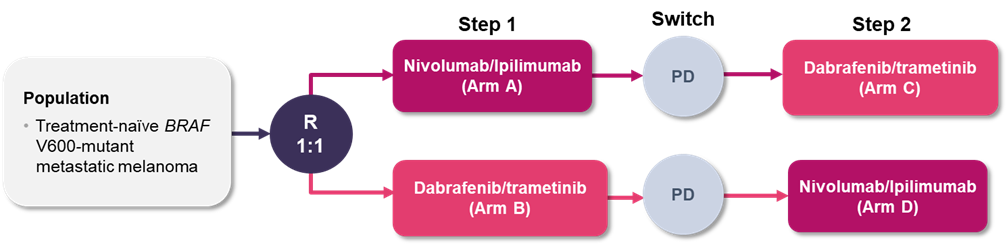
Abbreviations: PD, progressive disease; R, randomization.
After the fourth interim analysis, the data and safety monitoring committee and Cancer Therapy Evaluation Program recommended halting enrollment in the trial due to efficacy.11 There were 265 patients enrolled (133 in Arm A and 132 in Arm B). As of July 2021 and a median follow-up duration of 27.7 months, 27 patients had switched to Arm C and 46 to Arm D. For patients whose treatment sequence began with nivolumab/ipilimumab, the 2-year overall survival rate was superior to those whose treatment sequence began with dabrafenib/trametinib (72% vs 52%, p=0.0095); the difference in survival curves began to diverge after 10 months of treatment. The median duration of response was not reached in Arm A and was 12.7 months in Arm B. The objective response rates (ORR) also revealed potential benefit from first-line nivolumab/ipilimumab therapy as the ORR in patients receiving first-line nivolumab/ipilimumab was 46% (Arm A), as compared to 30% who received second-line nivolumab/ipilimumab (Arm D). The ORR for first-line versus second-line dabrafenib/trametinib were similar (Arm B vs Arm C, 43% vs 48%). Grade 3 or higher toxicity was reported in 60% of patients in Arm A versus 52% in Arm B.
SECOMBIT
The ongoing SECOMBIT phase 2 trial also addressed the optimal sequence of ICIs and BRAF/MEK inhibitors in BRAF-mutated metastatic melanoma, with the inclusion of a third arm that evaluated the potential benefit of a short-course of BRAF/MEK inhibitor therapy followed by maintenance ICI therapy.12 Patients with treatment-naïve BRAF V600 mutant metastatic melanoma were enrolled and randomized to receive either nivolumab/ipilimumab, encorafenib/binimetinib, or 8-week treatment with encorafenib/binimetinib followed by nivolumab/ipilimumab (sandwich approach) until disease progression and were then assigned to the alternative therapy (Figure 2). The nivolumab/ipilimumab treatment arms received nivolumab/ipilimumab induction dosing every 3 weeks for 4 cycles followed by nivolumab 3 mg/kg every 2 weeks. The encorafenib/binimetinib arms received encorafenib 450 mg once daily plus binimetinib 45 mg twice daily. The primary outcome was overall survival.
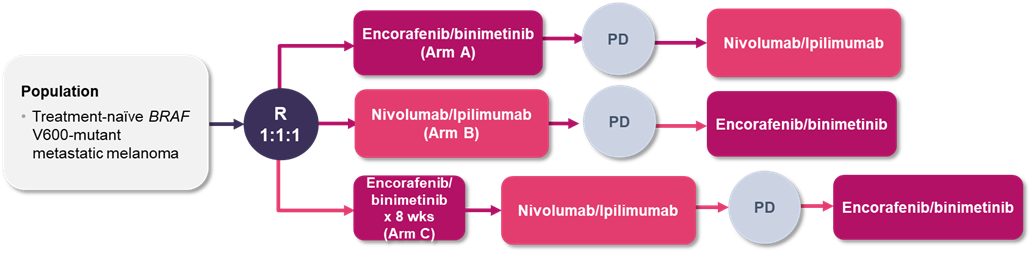
Abbreviations: PD, progressive disease; R, randomization.
A total of 251 patients were enrolled in the trial.12 Each arm within the study met its primary endpoint with at least 30 patients alive at 24 months; the median overall survival was not reached in any of the treatment arms (analyses are ongoing). The overall survival rates at 2 and 3 years were 65% and 54% in Arm A, 73% and 62% in Arm B, and 69% and 60% in Arm C, respectively. Total PFS rates at 2 years were 46%, 65%, and 57% in Arms A, B, and C. While this phase 2 trial did not plan any formal comparative tests, the study found a similar trend towards higher PFS and overall survival for those treated with initial ICIs followed by BRAF/MEK inhibitors.
Discussion
There are limited prospective evaluations available that guide the best initial treatment or the preferred treatment sequence in patients with BRAF V600-mutant metastatic melanoma. A recent phase 3 and phase 2 trial have been initially presented to help answer this clinical question.11,12 Unfortunately, these data are thus far only available as meeting abstracts and some data are not fully mature. Overall, however, these data appear to support use of initial ICIs over BRAF/MEK inhibitor combination therapy, with the potential to use a sandwich approach to derive the benefits of a quicker, deeper response with BRAF/MEK inhibitors combined with the durability of response with ICIs.11-13 The front-line use of ICIs may be further beneficial as the DREAMseq data suggested that ICIs were less effective after progression on targeted therapy, whereas ORRs with BRAF/MEK inhibitors were similar regardless of whether they were used as first-line or second-line therapy.11 Future research is needed to further clarify these optimal sequences, particularly with an increase in the use of systemic adjuvant treatment where the population with metastatic disease may be increasingly treatment-experienced, as well as the role of triplet therapies like the combination of vemurafenib, cobimetinib, and atezolizumab, along with others in phase 3 development.14
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
- Saginala K, Barsouk A, Aluru JS, Rawla P, Barsouk A. Epidemiology of melanoma. Med Sci (Basel). 2021;9(4):63. doi:10.3390/medsci9040063
- Rashid S, Tsao H. Recognition, staging, and management of melanoma. Med Clin North Am. 2021;105(4):643-661. doi:10.1016/j.mcna.2021.04.005
- SEER cancer stat facts: Melanoma of the skin. National Cancer Institute. Accessed January 19, 2022. https://seer.cancer.gov/statfacts/html/melan.html
- Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med. 2021;384(23):2229-2240. doi:10.1056/NEJMra2034861
- Trojaniello C, Luke JJ, Ascierto PA. Therapeutic advancements across clinical stages in melanoma, with a focus on targeted immunotherapy. Front Oncol. 2021;11:670726. doi:10.3389/fonc.2021.670726
- NCCN clinical practice guidelines in oncology, melanoma: cutaneous. Version 2.2022. National Comprehensive Cancer Network. Updated January 26, 2022. Accessed March 24, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- Mitchell TC, Karakousis G, Schuchter L. Melanoma. In: Niederhuber JE, Armitage JO, Kastan MB, Doroshow JH, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Elsevier; 2020.
- Teixido C, Castillo P, Martinez-Vila C, Arance A, Alos L. Molecular markers and targets in melanoma. Cells. 2021;10(9):2320. doi:10.3390/cells10092320
- Seth R, Messersmith H, Kaur V, et al. Systemic therapy for melanoma: ASCO guideline. J Clin Oncol. 2020;38(33):3947-3970. doi:10.1200/jco.20.00198
- Atkins MB, Lee SJ, Chmielowski B, et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J Clin Oncol. 2021;39(36_suppl):356154-356154. doi:10.1200/JCO.2021.39.36_suppl.356154
- Ascierto PA, Mandala M, Ferrucci PF, et al. LBA40 SECOMBIT: The best sequential approach with combo immunotherapy [ipilimumab (I) /nivolumab (N)] and combo target therapy [encorafenib (E)/binimetinib (B)] in patients with BRAF mutated metastatic melanoma: A phase II randomized study. Annals of Oncology. 2021;32:S1316-S1317. doi:10.1016/j.annonc.2021.08.2118
- Ziogas DC, Konstantinou F, Bouros S, Gogas H. Identifying the optimum first-line therapy in BRAF-mutant metastatic melanoma. Expert Rev Anticancer Ther. 2020;20(1):53-62. doi:10.1080/14737140.2020.1711737
- Dummer R, Ascierto PA, Nathan P, Robert C, Schadendorf D. Rationale for immune checkpoint inhibitors plus targeted therapy in metastatic melanoma: A review. JAMA Oncol. 2020;6(12):1957-1966. doi:10.1001/jamaoncol.2020.4401
Prepared by:
Samantha Spencer, PharmD, BCPS
Clinical Assistant Professor
University of Illinois at Chicago College of Pharmacy
April 2022
The information presented is current as 03/24/2022. This information is intended as an educational piece and should not be used as the sole source for clinical decision-making.